Abstract
Background: Patients (pt) with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) who are elderly, or have secondary AML (sAML), or relapsed/refractory (R/R) disease have poor outcomes. Venetoclax (VEN), an oral BCL2 inhibitor, has shown activity in R/R AML as single-agent and in combination with hypomethylating agents (HMA) in newly diagnosed unfit AML. We designed a phase II trial to evaluate the safety and efficacy of VEN with 10-days (D) of decitabine (DEC) in AML and high risk MDS.
Methods: Eligible AML pts included those who had failed prior therapy, or were newly diagnosed (ND) elderly pts (>60 years), or had sAML. ECOG score ≤3, WBC count ≤10 x109/L, and adequate organ function were required. VEN was given on day 1-28 in cycle (cy) 1 and D1-21 in cy 2 onwards; and was interrupted on C1D21 if the 21D bone marrow showed clearance of blasts, until count recovery. VEN was dosed 200 mg PO daily (50% dose reduction) in pts needing CYP3A4 inhibitors. DEC was given 20 mg/m2 IVdaily on D1-10 until CR/CRi, followed by 5-day cycles. Hydroxyurea or ara-C could be used for cytoreduction prior to starting therapy. Prophylactic antimicrobials were used until neutrophil recovery. Tyrosine kinase inhibitors could be used in applicable patients. Primary objective was to determine overall response rate (ORR) including complete remission (CR), CR with incomplete blood count recovery (CRi), partial remission (PR), and morphologic leukemia-free state in pts with AML. Secondary objectives were to determine safety of the combination; duration of response (DOR), disease-free survival (DFS) and overall survival (OS).
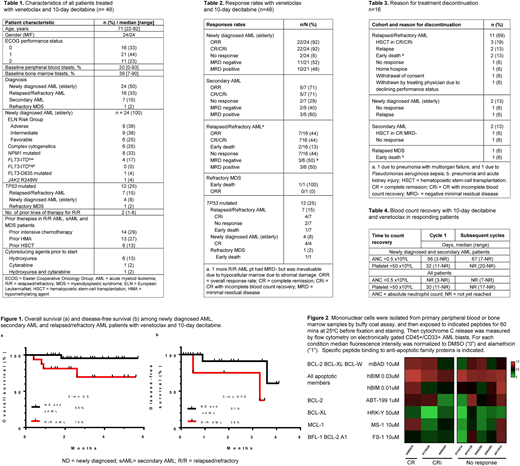
Results: 48 pts were enrolled between January and May 2018 (Table 1). 24 pts (50%) had ND AML, 7 pts (15%) had sAML, and 16 pts (33%) had R/R AML. Prior therapies are listed in Table 1. The overall CR/CRi rate was 71% (34/48). CR/CRi rate for ND, sAML and R/R AML were 92%, 71% and 44%, respectively (Table 2). Negative minimal residual disease (MRD-) by flow cytometry at the time of response was achieved in 16/33 responding pts (49%). CR/CRi with MRD- was achieved in 11/21 pts with ND AML (52%), 2/5 pts with sAML (40%), and 3/6 pts w R/R AML (50%). CR/CRi rate in TP53 mutated pts was 67% (8/12, Table 2). Additional therapies included ponatinib in 1 pt with AML and t(9;22) who achieved a CRi; and sorafenib in 5 pts (4 FLT3-ITD, 1 FLT3 S749L variant) of which 2 ITD pts achieved CRi and 3 pts did not respond. Median time to first response was 43D (range 20-110) with a median of 1 cy to best response (range 1-3). At a median follow-up of 2.3 months (mo; range 1.4-5.7), pts had received a median of 2 cy (range 2-5) and 32 pts continue on study. Reasons for discontinuation are shown in Table 3. Median OS has not been reached (NR) for ND and sAML pts (NR, range 1.8 mo-NR) and R/R AML (NR, range 0.4 mo-NR, Fig 1a). Median DFS (Fig 1b) and DOR for ND and sAML pts are also NR (range 0.9 mo-NR). Median DFS and DOR for R/R AML pts were 3.3 mo (range 0.5-NR). 10 pts received GCSF. 59 treatment-emergent adverse events (TEAE) occurred in 31 pts, out of which 48 were grade (gr) 3/4. The most frequent gr 3/4 TEAE were infections, with gr 3/4 neutropenia (53%), febrile neutropenia (14%), and tumor lysis syndrome (TLS, n=2, 4%). 1 pt with WBC count 12 x109/L developed TLS on C1D2 which resolved with rasburicase and holding VEN; another pt with WBC count 28 x109/L developed TLS on C1D2 needing hemodialysis for 12 days, prompting study amendment to the current baseline WBC≤10 x109/L. Time to blood count recovery are shown in Table 4. There were total 6 deaths, all in pts with R/R AML (n=5) and treated sAML (n=1), including 3 deaths in hospice, 2 early deaths in relapsed AML pts due to infection; and 1 early death in a relapsed MDS pt due to pneumonia and acute kidney injury unrelated to therapy. There were no deaths in the ND AML pts. 30D and 60D mortality rates were 8% and 10%, respectively. Preliminary BH3 profiling data in R/R cohort showed BCL-2 priming (by assessing cytochrome C release to recombinant BAD peptide and ABT-199) in 7/8 pts irrespective of their response; however, pts who failed to achieve CR/CRi demonstrated co-dependence on other anti-apoptotic proteins MCL-1, BCL-XL and A1 (Fig 2). Additional BH3 profiling and CyTOF analyses are ongoing.
Conclusion: The DEC10-VEN regimen had an acceptable safety profile and excellent response rates with CR/CRi of 92% in ND AML, 71% in sAML, and 44% in R/R AML with MRD- in 52% of ND AML, 40% of sAML and 50% of R/R AML. Trial is continuing to accrue (NCT03404193).
Maiti:Celgene Corporation: Other: Research funding to the institution. DiNardo:AbbVie: Consultancy, Other: Advisory role; Agios: Consultancy, Other: Advisory role; Bayer: Other: Advisory role; Celgene: Other: Advisory role; Medimmune: Other: Advisory role; Karyopharm: Other: Advisory role. Cortes:Novartis: Consultancy, Research Funding; Arog: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Pemmaraju:plexxikon: Research Funding; novartis: Research Funding; Affymetrix: Research Funding; samus: Research Funding; cellectis: Research Funding; celgene: Consultancy, Honoraria; SagerStrong Foundation: Research Funding; abbvie: Research Funding; daiichi sankyo: Research Funding; stemline: Consultancy, Honoraria, Research Funding. Kadia:Celgene: Research Funding; Amgen: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Takeda: Consultancy; BMS: Research Funding; BMS: Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Research Funding; Abbvie: Consultancy; Jazz: Consultancy, Research Funding; Abbvie: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Pfizer: Consultancy, Research Funding. Ravandi:Amgen: Honoraria, Research Funding, Speakers Bureau; Abbvie: Research Funding; Bristol-Myers Squibb: Research Funding; Jazz: Honoraria; Sunesis: Honoraria; Abbvie: Research Funding; Xencor: Research Funding; Sunesis: Honoraria; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Xencor: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Jazz: Honoraria; Bristol-Myers Squibb: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria. Short:Takeda Oncology: Consultancy. Daver:BMS: Research Funding; ImmunoGen: Consultancy; Incyte: Consultancy; Otsuka: Consultancy; Daiichi-Sankyo: Research Funding; Incyte: Research Funding; Novartis: Consultancy; Sunesis: Research Funding; Karyopharm: Research Funding; Pfizer: Research Funding; Alexion: Consultancy; Karyopharm: Consultancy; Pfizer: Consultancy; Sunesis: Consultancy; Kiromic: Research Funding; ARIAD: Research Funding; Novartis: Research Funding. Sasaki:Otsuka Pharmaceutical: Honoraria. Thompson:Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jain:Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; ADC Therapeutics: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Pharmacyclics: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Infinity: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Genentech: Research Funding; Verastem: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Pfizer: Research Funding; Cellectis: Research Funding; Verastem: Research Funding; BMS: Research Funding; Servier: Research Funding; Infinity: Research Funding; Astra Zeneca: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Incyte: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jabbour:Pfizer: Consultancy, Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding. Andreeff:AstraZeneca: Research Funding. Konopleva:Stemline Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal